What You Need to Know About Sativex (Pharmaceutical Cannabis Drug)
Many are starting to hear about a marijuana-based pharmaceutical called Sativex. The drug, which comes as an oral spray, has already been approved in 24 countries as a treatment for multiple sclerosis.
While yet to be approved in the United States, Sativex is legally available in Canada, Australia, Britain and, most recently, France. But the drug has generated a lot of talk in the USA, largely due to its role in the medical marijuana debate.
According to a recent interview with GW Pharmaceuticals, the company behind the drug, Sativex could be just months away from FDA approval. And if that happens, there’s no doubt the debate over marijuana as a medicine will only intensify. Below are a few facts about Sativex that should be considered:
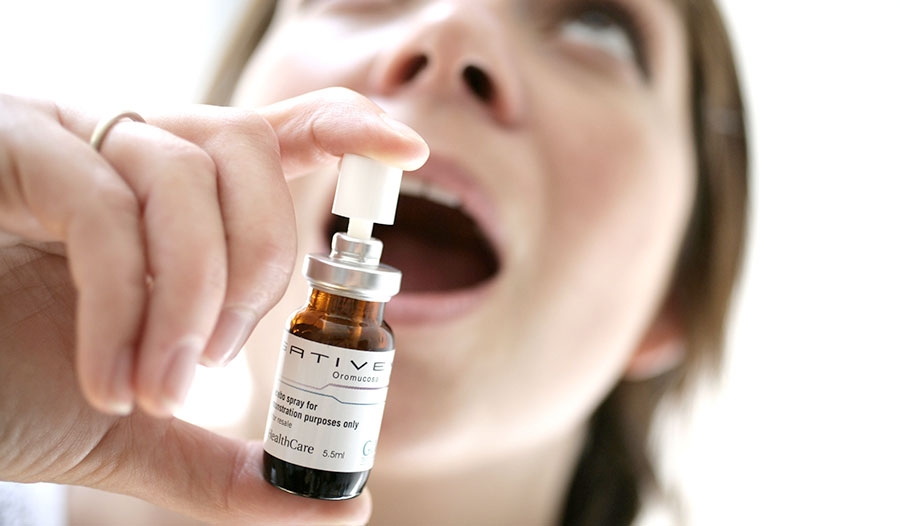
Sativex is a marijuana extract.
Despite its pharmaceutical sounding name, Sativex is made from the cannabis plant itself. GW Pharmaceuticals CEO Geoffrey Guy has noted to the press that Sativex contains all of the same compounds found in cannabis.
The difference, however, is that Sativex is manufactured using modern processes that allow the medicine to be standardized – meaning that each bottle of the spray contains the same concentration of active ingredients. The formula for Sativex includes a 1:1 equal ratio of THC to CBD.
A lack of standardization happens to be one of the main sticking points for doctors who hesitate to support medical marijuana.
Sativex is made in a country where cannabis is illegal.
Sativex is developed and manufactured in the UK, where GW Pharmaceuticals is based.
Despite the fact that marijuana remains illegal in the UK, even for medical purposes, the government issued a license to GW Pharmaceuticals in 1998 allowing the company to grow cannabis for the development of new medicines.
Currently, Sativex is an approved treatment in the UK. At the same time, advocacy groups are still fighting to have medical marijuana legalized in the country.
Sativex has all the same side effects as marijuana.
Since Sativex is made from cannabis, it also produces a high. While the ratio of THC to CBD may be less than that of more potent cannabis strains, Health Canada’s Fact Sheet warns that Sativex can still cause “symptoms of cannabinoid intoxication.â€
Dizziness and fatigue are also the most commonly reported side effects of taking Sativex. And like cannabis, studies have confirmed that Sativex has no permanent effect on cognitive function.
On the other hand, Sativex is administered as a mouth spray and has been found to cause oral discomfort in 20-25% of patients. Medical marijuana users do not typically experience this problem and often rely on vaporizers to avoid the negative effects of smoking.
Sativex could hit USA pharmacies as early as this year.
Sativex has been gradually making its way through the FDA approval process. It is currently in Phase III trials, the final phase of clinical trials, as a treatment for cancer pain.
GW Pharmaceuticals says it expects to receive final FDA approval within this year or the next. Once approved, the drug could be prescribed by doctors and sold in pharmacies across the USA
Sativex is staggeringly expensive.
The cost of Sativex in countries where it is approved has already proven to be a barrier.
In New Zealand, an average annual prescription of Sativex costs about USD16,000. Professor Gavin Giovannoni of The London School of Medicine also notes that “Sativex has not been proven to be cost-effective” in the UK, which has led “a large number” of MS patients to continue using illegal forms of cannabis.
Still, companies face significant costs associated with obtaining clinical data that will satisfy health authorities. These costs often factor into the price that patients pay.
Sativex is not considered cannabis under USA federal law.
While some might think that FDA approval of Sativex would make it impossible for the USA government to continue defending its classification of cannabis as a Schedule I drug, it may not be that difficult after all.
As it turns out, Sativex has been given its own scientific name: nabiximols. Like other marijuana-based medicines, such as dronabinol (Marinol) and nabilone (Cesamet), this allows Sativex to be scheduled separately from cannabis.
Most countries where Sativex is sold have used this approach in order to maintain a ban on medical marijuana.
Sativex may be useful for more than multiple sclerosis.
While Sativex is currently approved solely for the treatment of MS-related muscle spasticity, the drug is also being trialled for a number of other conditions.
Besides the company’s cancer pain trials in the USA, Sativex is being studied in the UK as an add-on treatment for gliomas (brain tumors). Previous studies have also suggested benefits in treating arthritis and neuropathic pain.
March 2014




